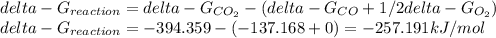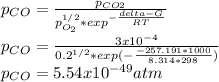Chemistry, 06.05.2020 00:21 Jenniferojeda2002
Hat is the pressure of CO(g) in equilibrium with the CO2(g) and O2(g) in the atmosphere at 25 C? The partial pressure of O2(g) is 0.2 bar and the partial pressure of CO2(g) is 3 * 10-4 bar. CO is extremely poisonous because it forms a very strong complex with hemoglobin. Should you worry?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
Hat is the pressure of CO(g) in equilibrium with the CO2(g) and O2(g) in the atmosphere at 25 C? The...
Questions

Mathematics, 16.04.2020 22:46


Mathematics, 16.04.2020 22:46

Geography, 16.04.2020 22:46

Mathematics, 16.04.2020 22:46

Mathematics, 16.04.2020 22:46

History, 16.04.2020 22:46


English, 16.04.2020 22:46

Mathematics, 16.04.2020 22:46


English, 16.04.2020 22:46




Mathematics, 16.04.2020 22:46



Mathematics, 16.04.2020 22:46