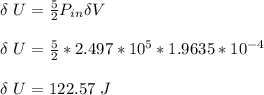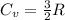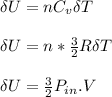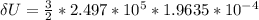Chemistry, 06.05.2020 02:07 isaihcarter
0.0500 mol of gas occupies a cylinder which is sealed on top by a moveable piston. The piston is circular, with a mass of 30.0 kg and diameter of 5.00 cm. It is supported only by the pressure of the gas in the cylinder. Outside of the cylinder, there is air at 1.00 atm. Initially, the piston is 30.0 cm above the bottom of the cylinder. Heat is then added, causing the gas to expand until the piston is 40.0 cm above the bottom of the cylinder. For this process, find the change in internal energy, the work done by the gas, and the heat which flows into the gas.
a. assuming that the gas is N2.
b. assuming that the gas is neon

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1



Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3
You know the right answer?
0.0500 mol of gas occupies a cylinder which is sealed on top by a moveable piston. The piston is cir...
Questions









Computers and Technology, 13.07.2021 16:30


Advanced Placement (AP), 13.07.2021 16:30


English, 13.07.2021 16:30


Computers and Technology, 13.07.2021 16:30





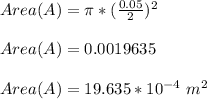
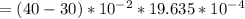

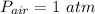 = 101325 Pa
= 101325 Pa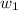 = PΔV
= PΔV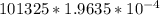
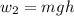

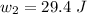
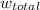 for both cases is:
for both cases is: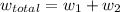
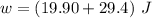
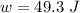
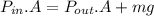
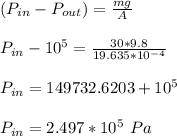
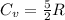
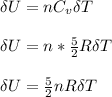
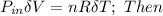 ;
;