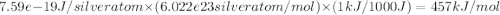The work function (ϕ) of a metal is the minimum energy needed to remove an electron from its surface. (a) Is it easier to remove an electron from a gaseous silver atom or from the surface of solid silver (ϕ = 7.59×10−19 J; IE = 731 kJ/mol)? (b) Explain the results in terms of the electron-sea model of metallic bonding.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 23.06.2019 10:50
Gene expression control that occurs during the generation of rna is a. controlled at transcription b. control before transcription c. controlled after transcription d. controlled after translation
Answers: 3

Chemistry, 23.06.2019 14:20
Neutral atoms of argon, atomic number 18, have the same number of electrons as each of the following items except: cl- s -2 k+ ca +2 ne
Answers: 2
You know the right answer?
The work function (ϕ) of a metal is the minimum energy needed to remove an electron from its surface...
Questions

History, 26.04.2020 03:51

Mathematics, 26.04.2020 03:51

Mathematics, 26.04.2020 03:51



French, 26.04.2020 03:51

Mathematics, 26.04.2020 03:51

Biology, 26.04.2020 03:51

Chemistry, 26.04.2020 03:51





Social Studies, 26.04.2020 03:51

English, 26.04.2020 03:51



Mathematics, 26.04.2020 03:51

Mathematics, 26.04.2020 03:51