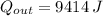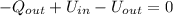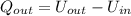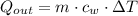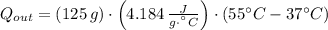Chemistry, 06.05.2020 01:57 jortizven0001
How much heat energy, in joules, is released to your body after you drink a cup of hot tea containing 125g of water that is cooled from 55℃ to a body temperature at 37℃ (water has a specific heat capacity of 4.184 J/g℃

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:40
Water ionizes by the equation h2o(l)⇌h+(aq)+oh−(aq) the extent of the reaction is small in pure water and dilute aqueous solutions. this reaction creates the following relationship between [h+] and [oh−]: kw=[h+][oh−] keep in mind that, like all equilibrium constants, the value of kw changes with temperature.
Answers: 1

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
You know the right answer?
How much heat energy, in joules, is released to your body after you drink a cup of hot tea containin...
Questions

English, 04.05.2021 16:50

Computers and Technology, 04.05.2021 16:50










Mathematics, 04.05.2021 16:50




Law, 04.05.2021 16:50

Mathematics, 04.05.2021 16:50

Mathematics, 04.05.2021 16:50