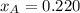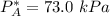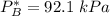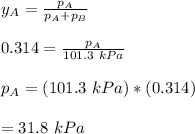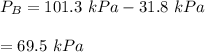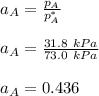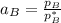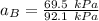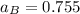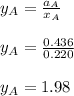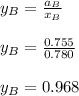Chemistry, 06.05.2020 03:07 rachel2005smith
By measuring the equilibrium between liquid and vapor phases of a binary solution at 30°C at 1atm, it was found that xA=0.220 when yA=0.314. Calculate the activities and activity coefficients of both components in the solution. The vapor pressures of the pure components at this temperature are 73kPa and 92.1kPa.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2
You know the right answer?
By measuring the equilibrium between liquid and vapor phases of a binary solution at 30°C at 1atm, i...
Questions





Mathematics, 11.05.2021 21:50


Business, 11.05.2021 21:50

Mathematics, 11.05.2021 21:50

Biology, 11.05.2021 21:50

Chemistry, 11.05.2021 21:50


Mathematics, 11.05.2021 21:50




Biology, 11.05.2021 21:50

Chemistry, 11.05.2021 21:50

History, 11.05.2021 21:50

Mathematics, 11.05.2021 21:50

Mathematics, 11.05.2021 21:50

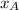 is the mole fraction in the liquid
is the mole fraction in the liquid 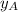 is the mole fraction in the vapor
is the mole fraction in the vapor