
Chemistry, 06.05.2020 04:33 bobbyhsu3751
Suppose a 500.mL flask is filled with 1.9mol of NO3 and 1.6mol of NO. The following reaction becomes possible: NO3gNOg 2NO2g The equilibrium constant K for this reaction is 8.33 at the temperature of the flask. Calculate the equilibrium molarity of NO. Round your answer to two decimal places

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
Suppose a 500.mL flask is filled with 1.9mol of NO3 and 1.6mol of NO. The following reaction becomes...
Questions



Mathematics, 19.02.2020 00:25


History, 19.02.2020 00:25

Computers and Technology, 19.02.2020 00:25







Mathematics, 19.02.2020 00:25




Mathematics, 19.02.2020 00:25



Mathematics, 19.02.2020 00:25

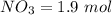
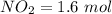
![[NO_3] = \frac{number \ of \ moles}{volume}](/tpl/images/0646/3223/b324f.png)
![[NO_3] = \frac{1.9}{0.500}](/tpl/images/0646/3223/e2e7e.png)
![[NO_3] = 3.8 \ M](/tpl/images/0646/3223/8a5ca.png)
![[NO_2] = \frac{number \ of \ moles}{volume}](/tpl/images/0646/3223/1e0e1.png)
![[NO_2] = \frac{}{} \frac{1.6}{0.500}](/tpl/images/0646/3223/24dfd.png)
![[NO_2] = 3.2 \ M](/tpl/images/0646/3223/096c8.png)
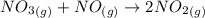
![K_c=\frac{[NO_2]^2}{[NO_3][NO]}](/tpl/images/0646/3223/498af.png)
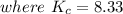
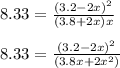
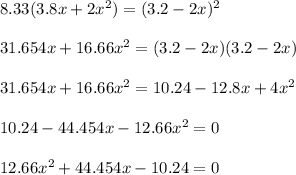
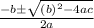
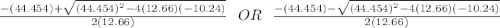
![[NO_3] = 3.8 +x = 3.8 + 0.21695](/tpl/images/0646/3223/7da74.png)
![[NO_2] = 3.2 +x = 3.2 + 0.21695](/tpl/images/0646/3223/43cc9.png)


