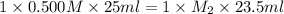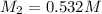Question 8
in a titration, 25.0 ml of 0.500 m khp is titrated to the equivalence point w...

Chemistry, 05.02.2020 07:51 emilyproce
Question 8
in a titration, 25.0 ml of 0.500 m khp is titrated to the equivalence point with naoh. the final solution volume is 48.5 ml. what is the value of m2 for the reaction?
0.256 m
0.532 m
0.970 m
0.500 m

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon? a balloon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1
You know the right answer?
Questions

Physics, 20.04.2021 23:20




Mathematics, 20.04.2021 23:20


Mathematics, 20.04.2021 23:20

Mathematics, 20.04.2021 23:20





Mathematics, 20.04.2021 23:20

Mathematics, 20.04.2021 23:20

History, 20.04.2021 23:20




English, 20.04.2021 23:20

Physics, 20.04.2021 23:20

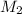 (concentration of NaOH) for the reaction will be, 0.532 M
(concentration of NaOH) for the reaction will be, 0.532 M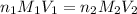
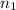 = basicity of an acid (KHP) = 1
= basicity of an acid (KHP) = 1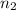 = acidity of a base (NaOH) = 1
= acidity of a base (NaOH) = 1
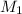 = concentration of
= concentration of 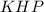 = 0.500 M
= 0.500 M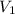 = volume of
= volume of 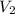 = volume of NaOH = 48.5 - 25 = 23.5 ml
= volume of NaOH = 48.5 - 25 = 23.5 ml
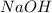 .
.