
Chemistry, 06.05.2020 03:59 michael1498
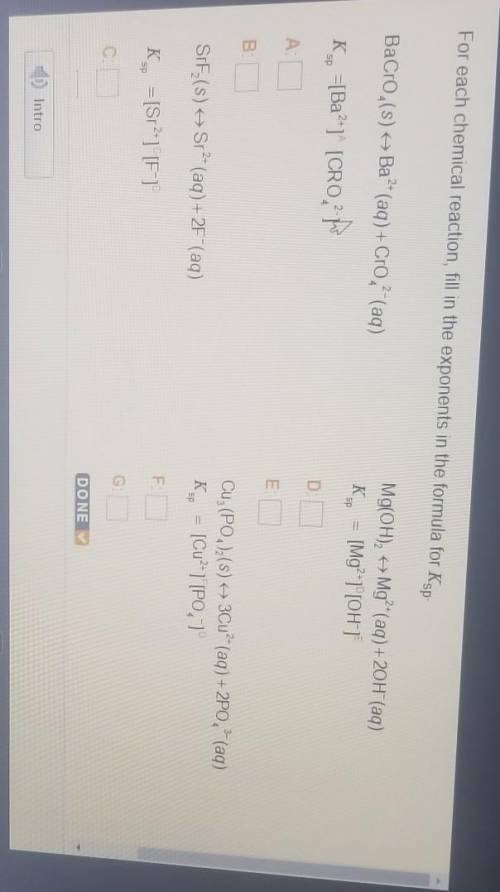
For each chemical reaction, fill in the exponents in the formula for Ks
BaCro (s) + Ba?- (aq) + Cro?- (aq)
K., =[Ba?"1" [CRO-
Mg(OH)2 + Mg²+(aq) + 20H (aq)
Ks = [Mg²+1"[OH]
SIF (S)
Sr- (aq) + 2F"(aq)
Cuz (PO4)2(s) + 3Cu?- (aq) + 2PO, 2 (aq)
Kg = [Cu²+ ][PO, 1
K., = [Sr2-1 [F-1

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3
You know the right answer?
For each chemical reaction, fill in the exponents in the formula for Ks
BaCro (s) + Ba?- (aq) +...
BaCro (s) + Ba?- (aq) +...
Questions

Mathematics, 13.04.2020 19:17


Mathematics, 13.04.2020 19:17

History, 13.04.2020 19:17


Mathematics, 13.04.2020 19:17




History, 13.04.2020 19:17




History, 13.04.2020 19:17


English, 13.04.2020 19:18

Mathematics, 13.04.2020 19:18



Mathematics, 13.04.2020 19:18



