Chemistry, 06.05.2020 05:27 leslie1811
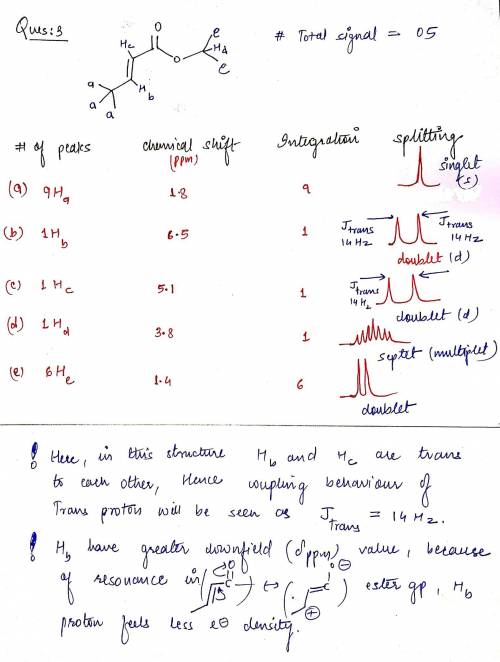
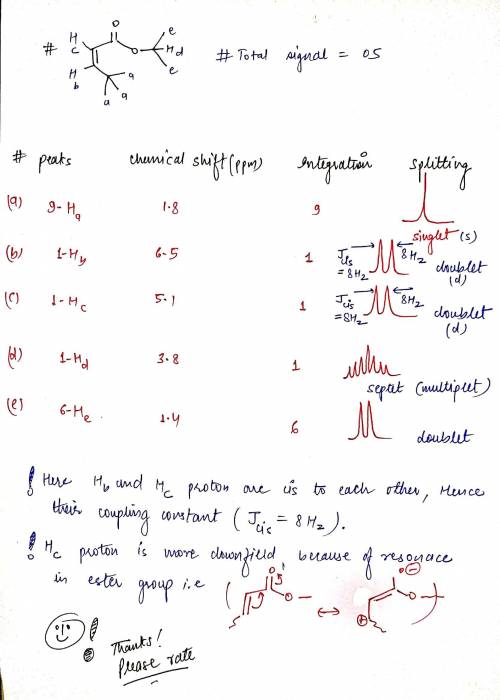
3. Consider the following stereoisomers of isopropyl methacrylate. Provide the expected number of peaks, the integrations of those peaks, the chemical shifts and the spliiting patterns expected for each. How are they different? b) Next to your description of the expected splitting pattern, please draw what that peak would look like. c) How could 1H NMR be used to determine the difference between these isomers? Please be specific.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3
You know the right answer?
3. Consider the following stereoisomers of isopropyl methacrylate. Provide the expected number of pe...
Questions

Mathematics, 15.11.2019 18:31


History, 15.11.2019 18:31





English, 15.11.2019 18:31


English, 15.11.2019 18:31



Mathematics, 15.11.2019 18:31

Mathematics, 15.11.2019 18:31


English, 15.11.2019 18:31

Mathematics, 15.11.2019 18:31



Mathematics, 15.11.2019 18:31