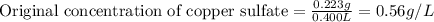Chemistry, 06.05.2020 05:05 Answers4833
Fe(s)+CuSO4(aq)Cu(s)+FeSO4(aq)*Note both 4's are subscripts and the equal signs represent an arrow. Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. He adds powdered iron to a 400.mL copper (II) sulfate sample from the plant until no more copper will precipitate. He then washes, dries, and weighs the precipitate, and finds that it has a mass of 89.mg .Calculate the original concentration of copper (II) sulfate in the sample. Round your answer to 2 significant digits. Answer in g/L.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3
You know the right answer?
Fe(s)+CuSO4(aq)Cu(s)+FeSO4(aq)*Note both 4's are subscripts and the equal signs represent an arrow....
Questions

Physics, 03.10.2021 01:00

Mathematics, 03.10.2021 01:00

Social Studies, 03.10.2021 01:00

Mathematics, 03.10.2021 01:00

Mathematics, 03.10.2021 01:00


English, 03.10.2021 01:00

Mathematics, 03.10.2021 01:00

Health, 03.10.2021 01:00


Social Studies, 03.10.2021 01:00

Social Studies, 03.10.2021 01:00

Social Studies, 03.10.2021 01:00


Physics, 03.10.2021 01:00


Computers and Technology, 03.10.2021 01:00

Mathematics, 03.10.2021 01:00

Chemistry, 03.10.2021 01:00

History, 03.10.2021 01:00

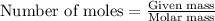 .....(1)
.....(1)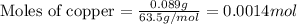
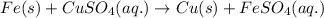
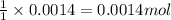 of copper sulfate
of copper sulfate