
Chemistry, 15.10.2019 18:40 dylankrenek
Butane, c4h10, reacts with oxygen, o2, to form water, h2o, and carbon dioxide, co2, as shown in the following chemical equation: 2c4h10(g)+13o2(g)-> 10h2o(g)+8co2(g)
calculate the mass of water produced when 1.77 grams of butane reacts with excessive oxygen?
calculate the mass of butane needed to produce 71.6 of carbon dioxide.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2
You know the right answer?
Butane, c4h10, reacts with oxygen, o2, to form water, h2o, and carbon dioxide, co2, as shown in the...
Questions


Social Studies, 08.11.2019 16:31


History, 08.11.2019 16:31

English, 08.11.2019 16:31

Mathematics, 08.11.2019 16:31

Mathematics, 08.11.2019 16:31


Social Studies, 08.11.2019 16:31

Mathematics, 08.11.2019 16:31


English, 08.11.2019 16:31

English, 08.11.2019 16:31

Mathematics, 08.11.2019 16:31

English, 08.11.2019 16:31




Spanish, 08.11.2019 16:31

Social Studies, 08.11.2019 16:31

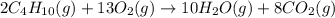
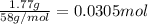
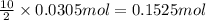 of water
of water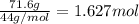
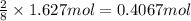 of butane
of butane

