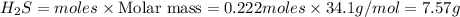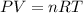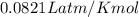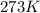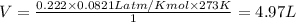Chemistry, 06.05.2020 06:12 harleyandpope90
If 25.0 grams of Sb2S3 reacts with an excess of hydrochloric acid, how many grams of H2S are formed? What volume does the H2S formed occupy under conditions of STP?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
You know the right answer?
If 25.0 grams of Sb2S3 reacts with an excess of hydrochloric acid, how many grams of H2S are formed?...
Questions

Advanced Placement (AP), 01.12.2020 01:10

Mathematics, 01.12.2020 01:10

Mathematics, 01.12.2020 01:10


English, 01.12.2020 01:10





History, 01.12.2020 01:10





History, 01.12.2020 01:10





Computers and Technology, 01.12.2020 01:10

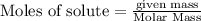
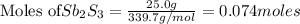
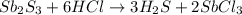
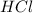 is the excess reagent,
is the excess reagent, 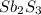 is the limiting reagent and it limits the formation of product.
is the limiting reagent and it limits the formation of product.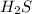
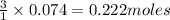 of
of