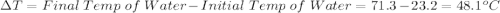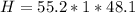Chemistry, 06.05.2020 06:05 fdougie111
You are running a calorimetry experiment where you are trying to determine the number of Calories (with a capital C!) in a peanut. You set up your aluminum can of water and take all your initial data, putting it in the table below. Then, you set your peanut ON FIRE You finish filling out your table once the peanut has gone out. How many Calories of heat did your peanut release? Round your answer to two digits after the decimal point.
Initial Mass of Peanut 3.11 grams
Final Mass of Peanut 0.52 grams
Mass of Water 55.2 grams
Initial Temp of Water 23.2 degrees C
Final Temp of Water 71.3 degrees C

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2


You know the right answer?
You are running a calorimetry experiment where you are trying to determine the number of Calories (w...
Questions

Mathematics, 10.05.2021 21:00


History, 10.05.2021 21:00

English, 10.05.2021 21:00

Mathematics, 10.05.2021 21:00


Mathematics, 10.05.2021 21:00

Mathematics, 10.05.2021 21:00












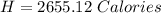
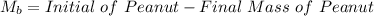
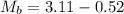
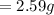
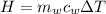
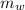 is the mass of water which is given as
is the mass of water which is given as 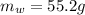
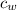 is the specific heat of water which has a constant value of
is the specific heat of water which has a constant value of 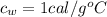
 is the change in temperature which can be evaluated as follows
is the change in temperature which can be evaluated as follows