Chemistry, 06.05.2020 05:58 CrsvrBryan7852
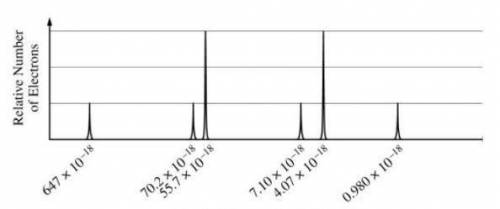
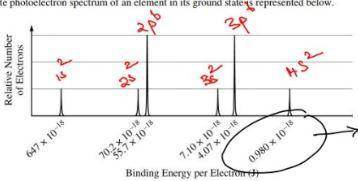
A) based on the spectrum, (i) write the ground-state electron configuration of the element, and (ii) identify the element. (b) calculate the wavelength, in meters, of electromagnetic radiation needed to remove an electron from the valence shell of an atom of the element.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 23.06.2019 03:30
Name atleast 3 type of energy associated with the microwave
Answers: 1
You know the right answer?
A) based on the spectrum, (i) write the ground-state electron configuration of the element, and (ii)...
Questions

Mathematics, 21.12.2020 14:30


Biology, 21.12.2020 14:30

French, 21.12.2020 14:30

World Languages, 21.12.2020 14:30

History, 21.12.2020 14:30

Computers and Technology, 21.12.2020 14:30




Chemistry, 21.12.2020 14:30

Computers and Technology, 21.12.2020 14:30

Mathematics, 21.12.2020 14:30

Chemistry, 21.12.2020 14:30

Biology, 21.12.2020 14:30

Mathematics, 21.12.2020 14:30


Chemistry, 21.12.2020 14:30

Mathematics, 21.12.2020 14:30

Mathematics, 21.12.2020 14:30