
Standard solution of FeSCN2+FeSCN2+ is prepared by combining 9.09.0 mL of 0.200.20 M Fe(NO3)3Fe(NO3)3 with 1.01.0 mL of 0.00200.0020 M KSCN. KSCN. The equation for the reaction is as follows. Fe(NO3)3+KSCN↽−−⇀FeSCN2++KNO3+2NO−3 Fe(NO3)3+KSCN↽−−⇀FeSCN2++KNO3+2NO3− What allows us to assume that the reaction goes essentially to completion? The reaction quotient Q is greater than Kc. Kc. The concentration of Fe(NO3)3Fe(NO3)3 is much higher than the concentration of KSCN. KSCN. The excess Fe3+Fe3+ prevents the formation of the neutral Fe(SCN)3.Fe(SCN)3. The equlibrium reaction has a very high Kc. Kc. Under the conditions given, Le Châtelier's principle dictates that the reaction shifts to the left. Based on that assumption, what is the equilibrium concentration of FeSCN2+?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
What is the relationship between wind and ocean waves? question 17 options: wind moving at higher speeds will transfer more energy to the water, resulting in stronger waves. wind moving at higher speeds will transfer energy over a larger part of the ocean water, resulting in waves with a shorter wavelength. winds moving at higher speeds with cause water to move forward at faster rates, causing larger ocean waves. winds moving at higher speeds will affect deeper water, resulting in waves that move at a faster rate. how do temperature and salinity affect deepwater currents? question 15 options: as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1


You know the right answer?
Standard solution of FeSCN2+FeSCN2+ is prepared by combining 9.09.0 mL of 0.200.20 M Fe(NO3)3Fe(NO3)...
Questions

Mathematics, 11.07.2019 20:30

English, 11.07.2019 20:30

Mathematics, 11.07.2019 20:30

History, 11.07.2019 20:30

Mathematics, 11.07.2019 20:30

Mathematics, 11.07.2019 20:30



Biology, 11.07.2019 20:30

Mathematics, 11.07.2019 20:30



Biology, 11.07.2019 20:30

Mathematics, 11.07.2019 20:30


Mathematics, 11.07.2019 20:30

Mathematics, 11.07.2019 20:30



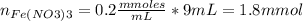
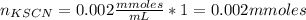
![[Fe(NO3)3]=\frac{1.8}{10} =0.18M](/tpl/images/0646/9896/2543f.png)
![[KSCN]=\frac{0.002}{10} =0.0002M](/tpl/images/0646/9896/5abd0.png)


