
Chemistry, 06.05.2020 07:41 punkinrichard1oxon2i
Suppose 0.829g of zinc chloride is dissolved in 100.mL of a 0.60M aqueous solution of potassium carbonate. Calculate the final molarity of chloride anion in the solution. You can assume the volume of the solution doesn't change when the zinc chloride is dissolved in it. Be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1


Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3
You know the right answer?
Suppose 0.829g of zinc chloride is dissolved in 100.mL of a 0.60M aqueous solution of potassium carb...
Questions



Mathematics, 30.01.2021 06:30


Mathematics, 30.01.2021 06:30

Mathematics, 30.01.2021 06:30


Mathematics, 30.01.2021 06:30

History, 30.01.2021 06:30

Mathematics, 30.01.2021 06:30

Physics, 30.01.2021 06:30


Mathematics, 30.01.2021 06:30

English, 30.01.2021 06:30

Mathematics, 30.01.2021 06:30


English, 30.01.2021 06:30

Mathematics, 30.01.2021 06:30


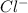 anion in solution is 0.122 M.
anion in solution is 0.122 M.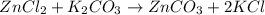
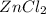 .
.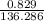 moles of
moles of 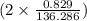 moles
moles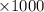
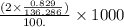 M
M


