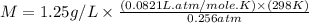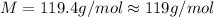Chemistry, 06.05.2020 07:30 Epicgible8136
Chloroform is a volatile liquid once commonly used in the laboratory but now being phased out due to its ozone depletion potential. If the pressure of gaseous chloroform in a flask is 195 mm Hg at 25°C and its density is 1.25 g/L, what is the molar mass of chloroform? A) 10.0 g/mol B) 76.3 g/mol C) 119 g/mol D) None of these

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
Chloroform is a volatile liquid once commonly used in the laboratory but now being phased out due to...
Questions

Mathematics, 05.08.2021 14:40

Computers and Technology, 05.08.2021 14:40

Computers and Technology, 05.08.2021 14:40

Chemistry, 05.08.2021 14:40


English, 05.08.2021 14:40

Mathematics, 05.08.2021 14:40


Mathematics, 05.08.2021 14:40


English, 05.08.2021 14:40

Mathematics, 05.08.2021 14:40

History, 05.08.2021 14:40

Social Studies, 05.08.2021 14:40


Spanish, 05.08.2021 14:40

Mathematics, 05.08.2021 14:40

English, 05.08.2021 14:50

Mathematics, 05.08.2021 14:50

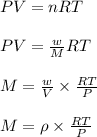
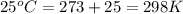
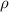 = density of gas = 1.25 g/L
= density of gas = 1.25 g/L