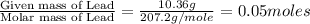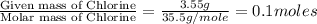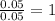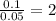Chemistry, 06.05.2020 07:29 jalexyinez
In an experiment similar to the zinc chloride experiment, a student placed a piece of lead in hydrochloric acid. Hydrogen gas was given off, and then the liquid was boiled off. The remaining solid, lead chloride was massed. Use the data below to determine the empirical formula of lead chloride.
mass of beaker 204.35 g
mass of lead and beaker before reaction 214.71 g
mass of lead chloride and beaker after reaction 218.26 g

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
This line graph compares the growth of plants that were kept in the sun for different amounts of time.a) on day 7, the plants kept in the sun for 3 hours were how tall? b) on day 7, the plants kept in the sun for 6 hours were how tall? c) on day 10, the plants kept in the sun for 9 hours were how tall? d) on day 11, the plant that was grown with 1 hour of sunlight was how tall? e) based on the graph, the plant grows best in what amount of sunlight?
Answers: 1

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
In an experiment similar to the zinc chloride experiment, a student placed a piece of lead in hydroc...
Questions

Biology, 27.12.2019 21:31


Mathematics, 27.12.2019 21:31

Mathematics, 27.12.2019 21:31

History, 27.12.2019 21:31



Mathematics, 27.12.2019 21:31






Social Studies, 27.12.2019 21:31


Mathematics, 27.12.2019 21:31


Social Studies, 27.12.2019 21:31


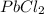
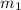 = 204.35 g
= 204.35 g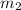 = 214.71 g
= 214.71 g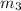 = 218.26 g
= 218.26 g = [214.71 - 204.35] = 10.36 g
= [214.71 - 204.35] = 10.36 g = [218.26 - 214.71] = 3.55 g
= [218.26 - 214.71] = 3.55 g