Chemistry, 06.05.2020 07:22 babyduckies37
The contents of a rock have a 206Pb to 238U mass ratio of 0.135:1.00. Assuming that the rock did not contain any 206Pb at the time of its formation, determine the age of the rock. Uranium-238 decays to lead-206 with a half-life of 4.5 billion years. Express the time to two significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1


Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
The contents of a rock have a 206Pb to 238U mass ratio of 0.135:1.00. Assuming that the rock did not...
Questions

Mathematics, 25.01.2020 18:31

Mathematics, 25.01.2020 18:31



Mathematics, 25.01.2020 18:31


Mathematics, 25.01.2020 18:31



Mathematics, 25.01.2020 18:31


Mathematics, 25.01.2020 18:31





Physics, 25.01.2020 18:31

History, 25.01.2020 18:31


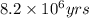
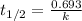
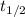 = half life of the reaction = 4.5 billion years =
= half life of the reaction = 4.5 billion years = 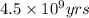
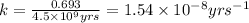
![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0647/7147/f1041.png)
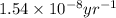
![[A_o]](/tpl/images/0647/7147/dc622.png) = initial amount of the sample = [1.00 + 0.135] = 1.135 g
= initial amount of the sample = [1.00 + 0.135] = 1.135 g