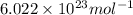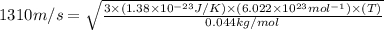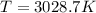Suppose that the root‑mean‑square velocity vrms of carbon dioxide molecules (molecular mass is equal to 44.0 g/mol ) in a flame is found to be 1310 m/s. What temperature T does this represent? The Boltzmann constant is k=1.38×10−23 J/K and Avogadro's number is A=6.022×1023 mol−1.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1
You know the right answer?
Suppose that the root‑mean‑square velocity vrms of carbon dioxide molecules (molecular mass is equal...
Questions




French, 17.09.2021 14:00

Mathematics, 17.09.2021 14:00

History, 17.09.2021 14:00


Health, 17.09.2021 14:00

Engineering, 17.09.2021 14:00



Health, 17.09.2021 14:00

Mathematics, 17.09.2021 14:00

Mathematics, 17.09.2021 14:00



Physics, 17.09.2021 14:00



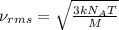
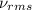 = root mean square speed = 1310 m/s
= root mean square speed = 1310 m/s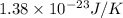
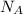 = Avogadro’s number =
= Avogadro’s number =