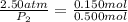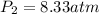Chemistry, 06.05.2020 07:10 tannercarr7428
Shaking up a soda bottles increase pressure by adding the number of gas particles (moles of carbon dioxide) into the inflexible can. If a soda bottle has an initial pressure of 2.50 atm and 0.150mol of CO2, then is shaken up so there are now 0.500 moles of carbon dioxide, what will the new pressure of the soda bottle be?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1


Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3
You know the right answer?
Shaking up a soda bottles increase pressure by adding the number of gas particles (moles of carbon d...
Questions

History, 19.05.2021 22:10


Mathematics, 19.05.2021 22:10

Chemistry, 19.05.2021 22:10

Mathematics, 19.05.2021 22:10


English, 19.05.2021 22:10



Mathematics, 19.05.2021 22:10

Mathematics, 19.05.2021 22:10

Physics, 19.05.2021 22:10



Mathematics, 19.05.2021 22:10


Arts, 19.05.2021 22:10


Mathematics, 19.05.2021 22:10

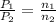
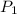 = initial pressure of gas = 2.50 atm
= initial pressure of gas = 2.50 atm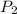 = final pressure of gas = ?
= final pressure of gas = ?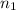 = initial number of moles of gas = 0.150 moles
= initial number of moles of gas = 0.150 moles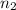 = final number of moles of gas = 0.500 mol
= final number of moles of gas = 0.500 mol