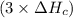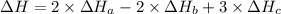2 Fe(s) + 6 HCl(aq) →2 FeCl3(aq) + 3 H2(g) ΔHa Fe2O3(s) + 6 HCl(aq) → 2 FeCI3(aq) + 3 H2O(l) ΔHb 2 H2(g) + O2(g) → 2 H2O(l) ΔHc Show how these equations must be summed together according to Hess's Law to determine ΔH for the combustion of iron (target equation shown below). Also show clearly how the ΔH values of each of the three reactions must be manipulated to determine the enthalpy of combustion of iron. 4 Fe(s) + 3 O2(g) → 2 Fe2O3(s) ΔH = ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1

Chemistry, 23.06.2019 09:10
In a 28 g serving of cheese curls there are 247mg of sodium. how much sodium is in a 12.5 ounce bag
Answers: 1
You know the right answer?
2 Fe(s) + 6 HCl(aq) →2 FeCl3(aq) + 3 H2(g) ΔHa Fe2O3(s) + 6 HCl(aq) → 2 FeCI3(aq) + 3 H2O(l) ΔHb 2 H...
Questions

Mathematics, 13.06.2020 12:57

Mathematics, 13.06.2020 12:57

Mathematics, 13.06.2020 12:57




French, 13.06.2020 12:57



Mathematics, 13.06.2020 12:57


Mathematics, 13.06.2020 12:57

Chemistry, 13.06.2020 12:57

Geography, 13.06.2020 12:57

Social Studies, 13.06.2020 12:57

Mathematics, 13.06.2020 12:57

History, 13.06.2020 12:57

Physics, 13.06.2020 12:57

English, 13.06.2020 12:57

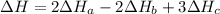
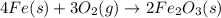
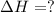
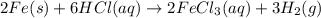 ;
; 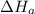
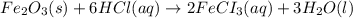 ;
; 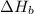
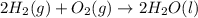 ;
; 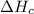
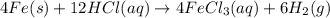 ;
; 
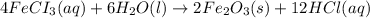 ;
; 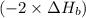
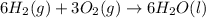 ;
;