
Chemistry, 17.10.2019 05:30 Blakemiller2020
All isotopes of a particular element have the same atomic number. how then do the isotopes of a particular element differ?
a. they have less or more protons.
b. they have less or more neutrons.
c. they have less or more electrons.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2


Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3
You know the right answer?
All isotopes of a particular element have the same atomic number. how then do the isotopes of a part...
Questions

Physics, 06.04.2021 18:10


Mathematics, 06.04.2021 18:10




History, 06.04.2021 18:10

English, 06.04.2021 18:10

Mathematics, 06.04.2021 18:10


Mathematics, 06.04.2021 18:10


Mathematics, 06.04.2021 18:10


Geography, 06.04.2021 18:10

History, 06.04.2021 18:10


Mathematics, 06.04.2021 18:10

Business, 06.04.2021 18:10

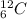 and
and 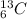 are isotopes. As both of them has 6 protons and there are 6 neutrons in
are isotopes. As both of them has 6 protons and there are 6 neutrons in 

