Chemistry, 06.05.2020 07:58 vaniavidal666
You will first investigate 5 diatomic molecules. Diatomic molecules are made up of 2 atoms.
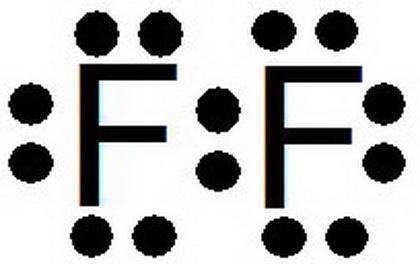
a. Select 2 fluorine atoms. How many valence electrons are in each fluorine atom?
b. Is a fluorine atom a metal or a non-metal?
c. Did the combination of these atoms create a covalent or ionic bond?
d. How are the valence electrons organized to form a bond between these atoms?
e. How is this different from the ionic bonds formed in the previous part of the activity?
f. What shape does this molecule form?
2.
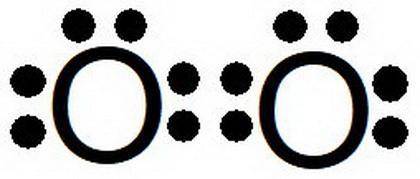
a. Select 2 oxygen atoms. How many valence electrons are in each oxygen atom?
b. Is an oxygen atom a metal or a non-metal?
c. Did the combination of these atoms create a covalent or ionic bond?
d. How are the valence electrons organized to form a bond between the atoms?
e. How is this bond different from the bond in the fluorine molecule in question 1?
f. What shape does this molecule form?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1


You know the right answer?
You will first investigate 5 diatomic molecules. Diatomic molecules are made up of 2 atoms.
a....
a....
Questions

English, 12.12.2020 15:50

Biology, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50



German, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50

History, 12.12.2020 15:50




English, 12.12.2020 15:50


Chemistry, 12.12.2020 15:50

Spanish, 12.12.2020 15:50