
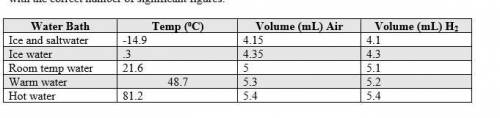
1. The actual value for absolute zero in degrees Celsius is −273.15. Use the formula below to determine your percent error for both gas samples.
|experimental value – actual value| x 100
actual value
2. If the atmospheric pressure in the laboratory is 1.2 atm, how many moles of gas were in each syringe? (Hint: Choose one volume and temperature pair from your data table to use in your ideal gas law calculation.)
Conclusion:
Write a conclusion statement that addresses the following questions:
• How did your experimental absolute zero value compare to the accepted value?
• Does your data support or fail to support your hypothesis (include examples)?
• Discuss any possible sources of error that could have impacted the results of this lab.
• How do you think the investigation can be explored further?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:50
If the mass of the products measured 120g what would the mass of the reactants a. 30g b. 60g c. 120g d. 240g
Answers: 1

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1
You know the right answer?
1. The actual value for absolute zero in degrees Celsius is −273.15. Use the formula below to determ...
Questions


Mathematics, 23.09.2020 08:01




Mathematics, 23.09.2020 08:01


Mathematics, 23.09.2020 08:01



Mathematics, 23.09.2020 08:01


Mathematics, 23.09.2020 08:01


Geography, 23.09.2020 08:01

Physics, 23.09.2020 08:01


Mathematics, 23.09.2020 08:01

English, 23.09.2020 08:01



