Chemistry, 25.04.2020 23:17 WonTonBagel
A helium sample occupies 1.50 L of space at 124.6 kPa. What pressure would the helium need to experience to have a volume of 0.75 L?
A. 130 kPa
B. 97 kPa
C. 250 kPa
D. 62 kPa

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1


Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
A helium sample occupies 1.50 L of space at 124.6 kPa. What pressure would the helium need to experi...
Questions

History, 05.05.2021 04:30




English, 05.05.2021 04:30

Mathematics, 05.05.2021 04:30

History, 05.05.2021 04:30

Mathematics, 05.05.2021 04:30


Mathematics, 05.05.2021 04:30




Mathematics, 05.05.2021 04:30





History, 05.05.2021 04:30

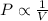
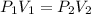
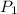 = initial pressure = 124.6 kPa
= initial pressure = 124.6 kPa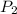 = final pressure = ?
= final pressure = ?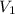 = initial volume = 1.50 L
= initial volume = 1.50 L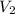 = final volume = 0.75 L
= final volume = 0.75 L