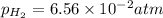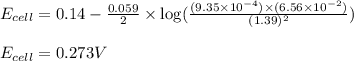Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2


Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
You know the right answer?
What is the calculated value of the cell potential at 298K for an
electrochemical cell with th...
electrochemical cell with th...
Questions


English, 20.10.2020 03:01

Mathematics, 20.10.2020 03:01

History, 20.10.2020 03:01










Mathematics, 20.10.2020 03:01

Chemistry, 20.10.2020 03:01


Mathematics, 20.10.2020 03:01

Mathematics, 20.10.2020 03:01


Geography, 20.10.2020 03:01

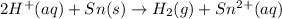
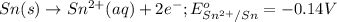
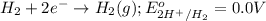
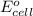 of the reaction, we use the equation:
of the reaction, we use the equation: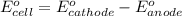
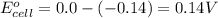
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Sn^{2+}]\times p_{H_2}}{[H^+]^2}](/tpl/images/0627/3491/76091.png)
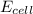 = electrode potential of the cell = ?
= electrode potential of the cell = ?![[H^{+}]=1.39M](/tpl/images/0627/3491/e38d3.png)
![[Sn^{2+}]=9.35\times 10^{-4}M](/tpl/images/0627/3491/dda0a.png)