Chemistry, 25.04.2020 04:49 jessieeverett432
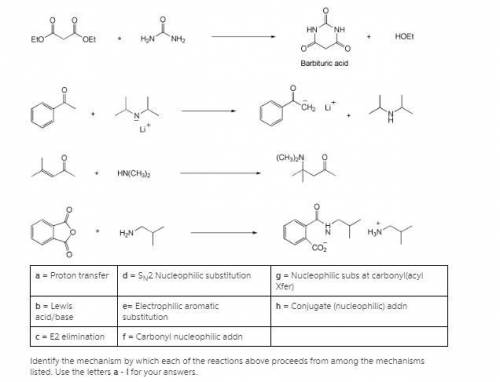
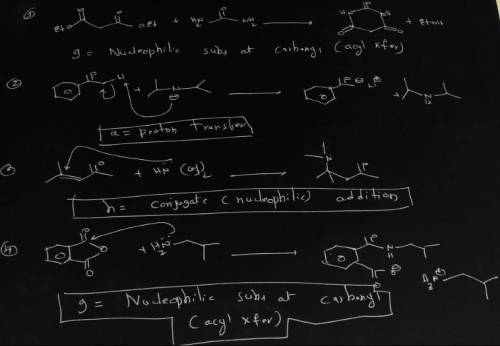
A = Proton transfer d = SN2 Nucleophilic substitution g = Nucleophilic subs at carbonyl(acyl Xfer) b = Lewis acid/base e= Electrophilic aromatic substitution h = Conjugate (nucleophilic) addn c = E2 elimination f = Carbonyl nucleophilic addn Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers. n(nh3)3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1

Chemistry, 23.06.2019 12:30
17) large amounts of very important metal titanium are made by reacting magnesium metal with titanium tetrachloride. titanium metal and magnesium chloride are produced. a) write the balanced equation for this reaction. b) how many kilograms of magnesium are required to produce 1.00 kilograms of titanium? ( show work, .)
Answers: 1

You know the right answer?
A = Proton transfer d = SN2 Nucleophilic substitution g = Nucleophilic subs at carbonyl(acyl Xfer) b...
Questions

History, 27.08.2019 13:00




Mathematics, 27.08.2019 13:00


Geography, 27.08.2019 13:00


Mathematics, 27.08.2019 13:00

Mathematics, 27.08.2019 13:00



History, 27.08.2019 13:00

History, 27.08.2019 13:00

Mathematics, 27.08.2019 13:00

Mathematics, 27.08.2019 13:00

Mathematics, 27.08.2019 13:00

History, 27.08.2019 13:00

Mathematics, 27.08.2019 13:00