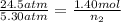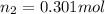A rigid tank contains 1.40 moles of an ideal gas. Determine the number of moles of gas that must be withdrawn from the tank to lower the pressure of the gas from 24.5 atm to 5.30 atm. Assume the volume of the tank and the temperature of the gas remain constant during this operation.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1


Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2
You know the right answer?
A rigid tank contains 1.40 moles of an ideal gas. Determine the number of moles of gas that must be...
Questions

English, 03.03.2022 23:00


Physics, 03.03.2022 23:00



Chemistry, 03.03.2022 23:00

Law, 03.03.2022 23:00

Spanish, 03.03.2022 23:10


Mathematics, 03.03.2022 23:10

Computers and Technology, 03.03.2022 23:10




Mathematics, 03.03.2022 23:10





Mathematics, 03.03.2022 23:10

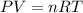
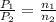
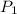 = initial pressure of gas = 24.5 atm
= initial pressure of gas = 24.5 atm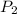 = final pressure of gas = 5.30 atm
= final pressure of gas = 5.30 atm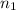 = initial number of moles of gas = 1.40 moles
= initial number of moles of gas = 1.40 moles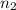 = final number of moles of gas = ?
= final number of moles of gas = ?