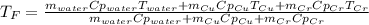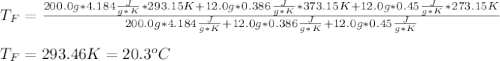Chemistry, 25.04.2020 04:09 hellodarkness14
A piece of copper (12.0 g) is heated to 100.0 °C. A piece of chromium (also 12.0 g) is chilled in an ice bath to 0 °C. The specific heat capacity of water is 4.184 J/g ⋅°C.
Both pieces of metal are placed in a beaker containing 200.0 g at 20.0 °C.
(a) Will the temperature of the water be greater than or less than 20.0 °C when thermal equilibrium is reached?
(b) Both pieces of metal are placed in a beaker containing 200.0 g at 20.0 °C. Calculate the final temperature.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
A piece of copper (12.0 g) is heated to 100.0 °C. A piece of chromium (also 12.0 g) is chilled in an...
Questions

Mathematics, 04.10.2019 21:30

History, 04.10.2019 21:30

Mathematics, 04.10.2019 21:30


Mathematics, 04.10.2019 21:30


History, 04.10.2019 21:30

Health, 04.10.2019 21:30

History, 04.10.2019 21:30

History, 04.10.2019 21:30




Mathematics, 04.10.2019 21:30






Mathematics, 04.10.2019 21:30

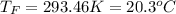
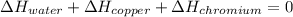
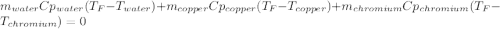 In such a way, by knowing that the heat capacities of copper and chromium are 0.386 and 0.45 J/(g°C) respectively, by solving for the equilibrium temperature one has:
In such a way, by knowing that the heat capacities of copper and chromium are 0.386 and 0.45 J/(g°C) respectively, by solving for the equilibrium temperature one has: