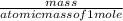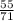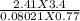Chemistry, 25.04.2020 03:24 smcculleymcculley
At what temperature will 55 grams of chlorine gas Cl2 exert a pressure of 245 kPa at a volume of 3.4 liters?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 20:00
Listenbase your answer to the question on the information below.nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body.cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment.which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2
You know the right answer?
At what temperature will 55 grams of chlorine gas Cl2 exert a pressure of 245 kPa at a volume of 3.4...
Questions

Mathematics, 14.01.2021 18:10

Mathematics, 14.01.2021 18:10



Mathematics, 14.01.2021 18:10




Mathematics, 14.01.2021 18:10

Computers and Technology, 14.01.2021 18:10



History, 14.01.2021 18:10


Mathematics, 14.01.2021 18:10

Chemistry, 14.01.2021 18:10

Mathematics, 14.01.2021 18:10



Mathematics, 14.01.2021 18:10