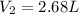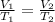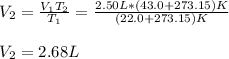Chemistry, 25.04.2020 02:59 Katepratt7637
Hot-air balloons rise because the hot air inside the balloon is less dense than the cooler air outside. Calculate the volume an air sample will occupy inside a balloon at 43.0C if it occupies 2.50L at the outside air temperature of 22.0C, assuming the pressure is the same at both locations

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

You know the right answer?
Hot-air balloons rise because the hot air inside the balloon is less dense than the cooler air outsi...
Questions

Geography, 09.10.2019 17:30

Spanish, 09.10.2019 17:30


English, 09.10.2019 17:30



Mathematics, 09.10.2019 17:30

Business, 09.10.2019 17:30




Mathematics, 09.10.2019 17:30



Physics, 09.10.2019 17:30

History, 09.10.2019 17:30


Mathematics, 09.10.2019 17:30

Mathematics, 09.10.2019 17:30