
Chemistry, 25.04.2020 03:00 destinywashere101
A chemist titrates 216.8 mL of a 0.3200 M butanoic acid (HC3H7CO2) solution with a 0.3200 M solution of NaOH. The titration setup shows that the butanoic acid solution is in the (Erlenmeyer) flask, and the NaOH solution is in the burette. The pKa of butanoic acid is 4.82. Calculate the pH of the solution in the flask after the chemist has added 231.4 mL of NaOH solution to it.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2


Chemistry, 22.06.2019 18:30
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2
You know the right answer?
A chemist titrates 216.8 mL of a 0.3200 M butanoic acid (HC3H7CO2) solution with a 0.3200 M solution...
Questions


Geography, 13.10.2020 17:01

Mathematics, 13.10.2020 17:01



Mathematics, 13.10.2020 17:01


Spanish, 13.10.2020 17:01


English, 13.10.2020 17:01


Mathematics, 13.10.2020 17:01

Mathematics, 13.10.2020 17:01


Mathematics, 13.10.2020 17:01

Mathematics, 13.10.2020 17:01


Mathematics, 13.10.2020 17:01


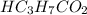 = 0.32 M,
= 0.32 M,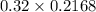
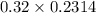
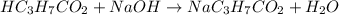
![[OH^{-}] = \frac{n}{V}](/tpl/images/0626/3976/691f7.png)
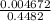
![-log [OH^{-}]](/tpl/images/0626/3976/337a4.png)


