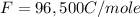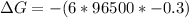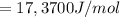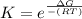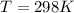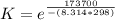Chemistry, 25.04.2020 03:33 madelynlittle5399
What is the value of the equilibrium constant at 25 oC for the reaction between the pair: Fe(s) and Cr3 (aq) to give Cr(s) and Fe2 (aq). Give your answer using E-notation with NO decimal places (e. g., 2 x 10-2 would be 2E-2; and 2.12 x 10-2 would also be 2E-2.). Do NOT include spaces, units, punctuation or anything else silly! Use the reduction potentials for Cr3 (aq) is -0.74 V and for Fe2 (aq) is -0.440 V. [a]

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3
You know the right answer?
What is the value of the equilibrium constant at 25 oC for the reaction between the pair: Fe(s) and...
Questions

Mathematics, 20.10.2020 18:01

English, 20.10.2020 18:01

Business, 20.10.2020 18:01

English, 20.10.2020 18:01


History, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01

Chemistry, 20.10.2020 18:01



English, 20.10.2020 18:01

Computers and Technology, 20.10.2020 18:01

Computers and Technology, 20.10.2020 18:01

Social Studies, 20.10.2020 18:01



Chemistry, 20.10.2020 18:01



Geography, 20.10.2020 18:01

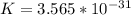
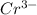 =
= 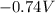
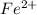 =
= 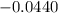
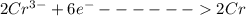
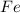 is oxidized to
is oxidized to 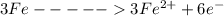
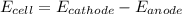
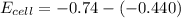
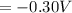
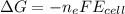
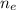 is the number of moles of electron which is 6
is the number of moles of electron which is 6