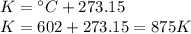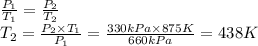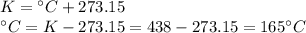Chemistry, 25.04.2020 00:33 ineedhelp2285
At a constant volume, a gas exerts a pressure of 660 kPa at 602 ºC. At what temperature, in Celsius, will the pressure reduce to 330 kPa?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1


Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
You know the right answer?
At a constant volume, a gas exerts a pressure of 660 kPa at 602 ºC. At what temperature, in Celsius,...
Questions




Mathematics, 30.05.2020 04:58



Mathematics, 30.05.2020 04:58



Mathematics, 30.05.2020 04:58

Mathematics, 30.05.2020 04:58

Biology, 30.05.2020 04:58



Advanced Placement (AP), 30.05.2020 04:58

History, 30.05.2020 04:58


Biology, 30.05.2020 04:58

History, 30.05.2020 04:58

Mathematics, 30.05.2020 04:58