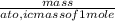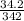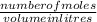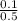Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1
You know the right answer?
What is the molarity of a sucrose solution containing 34.2 grams of sucrose in 500.0 mL of water? Th...
Questions

Geography, 25.01.2020 18:31

Mathematics, 25.01.2020 18:31


Mathematics, 25.01.2020 18:31

English, 25.01.2020 18:31

History, 25.01.2020 18:31

History, 25.01.2020 18:31

Mathematics, 25.01.2020 18:31




Mathematics, 25.01.2020 18:31



Biology, 25.01.2020 18:31

Biology, 25.01.2020 18:31



History, 25.01.2020 18:31

Biology, 25.01.2020 18:31