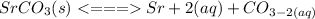Chemistry, 24.04.2020 18:19 brewerroserb
Consider the molar solubility of SrCO3 in 0.10 M Sr(NO3)2 versus in pure water. Ksp SrCO3 = 5.4 x 10-10 Which statement is true? A. The solubility of Sr(NO3)2 increases. B. The molar solubility of SrCO3 will be higher in 0.10 M 0 M Sr(NO3)2. C. The molar solubility of SrCO3 will be lower in water. D. The molar solubility of SrCO3 will be lower in 0.10 M 0 M Sr(NO3)2 because of Sr2+ E. There is not enough information given to determine an answer

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
Consider the molar solubility of SrCO3 in 0.10 M Sr(NO3)2 versus in pure water. Ksp SrCO3 = 5.4 x 10...
Questions



Mathematics, 30.10.2020 01:50

English, 30.10.2020 01:50


History, 30.10.2020 01:50


Mathematics, 30.10.2020 01:50

Mathematics, 30.10.2020 01:50





Mathematics, 30.10.2020 01:50



Mathematics, 30.10.2020 01:50


Biology, 30.10.2020 01:50

Mathematics, 30.10.2020 01:50

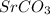 will be lower in 0.10 M 0 M
will be lower in 0.10 M 0 M 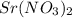 because of
because of 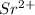 ) is the correct statement
) is the correct statement