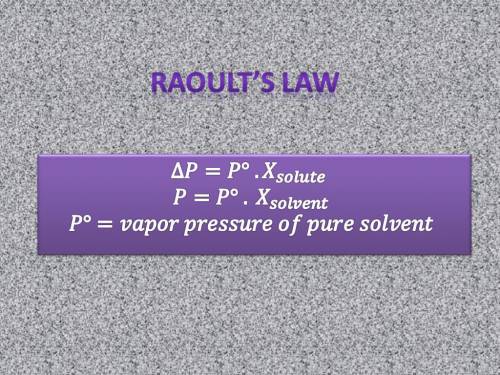
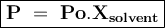Chemistry, 10.10.2019 22:00 cfnewton09
When two volatile liquids (x and y) are mixed, the solution process involves 1. breaking the intermolecular and attractions, and 2. forming new attractions.
complete this table describing how the relative strengths of these attractive forces affect vapor pressure and enthalpy of solution.
1. , , and are equal
2. is strongest
3. is weakest
raoult's law deviations & deltahsoln

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 05:00
Match the term to its description match term definition chemical energy a) internal energy caused by vibrations of atoms and molecules electrical energy b) electromagnetic energy that travels in waves radiant energy c) the movement of an electrical charge thermal energy d) potential energy stored in the bonds between atoms
Answers: 1
You know the right answer?
When two volatile liquids (x and y) are mixed, the solution process involves 1. breaking the intermo...
Questions




Health, 05.10.2019 16:00

Mathematics, 05.10.2019 16:00

History, 05.10.2019 16:00



Biology, 05.10.2019 16:00

Social Studies, 05.10.2019 16:00


Mathematics, 05.10.2019 16:00




Mathematics, 05.10.2019 16:00



History, 05.10.2019 16:00

Social Studies, 05.10.2019 16:00