
The hydrolysis of sucrose to glucose and fructose is catalyzed by the enzyme sucrase. The hydrolysis is first order in sucrose with a half-life of 4.8 103 s at 20°C. What fraction of sucrose remains after 2.0 hours? Please see Half Life of First Order Reactions for assistance. WebAssign will check your answer for the correct number of significant figures. .646 Incorrect: Your answer is incorrect. How long is required to hydrolyze 94% of the sucrose? WebAssign will check your answer for the correct number of significant figures. 54.1 Incorrect: Your answer is incorrect. hr

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5 m hcl? show all of the work needed to solve this problem. mg (s) + 2hcl (aq) → mgcl2 (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
The hydrolysis of sucrose to glucose and fructose is catalyzed by the enzyme sucrase. The hydrolysis...
Questions



Spanish, 05.02.2021 21:50


Social Studies, 05.02.2021 21:50



Mathematics, 05.02.2021 21:50




Mathematics, 05.02.2021 21:50


Mathematics, 05.02.2021 21:50

Chemistry, 05.02.2021 21:50


Mathematics, 05.02.2021 21:50

Mathematics, 05.02.2021 21:50

Mathematics, 05.02.2021 21:50

![rate=-\dfrac{d[A]}{dt}=k[A]](/tpl/images/0623/9914/df40f.png)
![\dfrac{[A]}{[A]_0}=e^{-kt}](/tpl/images/0623/9914/cabc4.png)
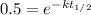
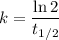
![\dfrac{A}{[A]_0}=\bigg(\dfrac{1}{2}\bigg)^n](/tpl/images/0623/9914/863b6.png)


