Chemistry, 24.04.2020 15:18 spoo262005
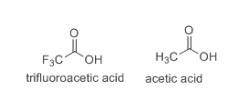
Which of the following item(s) explain the differences between the Ka values. Choose one or more: A. The negative charge is on the more electronegative fluorine atom in trifluoroacetate. B. The oxidation state for oxygen in trifluoroacetate is more negative than the oxidation state for oxygen in acetate. C. The trifluoroacetate molecule has more resonance structures than the acetate molecule. D. The electron-withdrawing fluorine atoms pull electron density from the oxygen in trifluoroacetate. The negative charge is more stabilized in trifluoroacetate by this effect.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:00
How has the scientific community addressed the safety of chemicals? a. chemicals are repeatedly tested, even those that have existed for a long time. b. existing chemicals are tested if they have never been tested before. c. chemicals are tested if they are suspected to have caused a problem. d. only new chemicals are tested.
Answers: 2

Chemistry, 21.06.2019 19:30
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

You know the right answer?
Which of the following item(s) explain the differences between the Ka values. Choose one or more: A....
Questions

Mathematics, 21.08.2020 20:01



History, 21.08.2020 20:01


Advanced Placement (AP), 21.08.2020 20:01



Mathematics, 21.08.2020 20:01


Mathematics, 21.08.2020 20:01

English, 21.08.2020 20:01



Geography, 21.08.2020 20:01


Mathematics, 21.08.2020 20:01

Computers and Technology, 21.08.2020 20:01