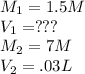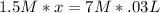Chemistry, 24.04.2020 06:39 brownvester44
PLS ANSWER ASAP, WILL GIVE BRAINLIEST ANSWER
Calculate the volume of 1.5 M nitric acid required to neutralize 30.0 mL of 7.0 M sodium hydroxide.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

You know the right answer?
PLS ANSWER ASAP, WILL GIVE BRAINLIEST ANSWER
Calculate the volume of 1.5 M nitric acid require...
Calculate the volume of 1.5 M nitric acid require...
Questions


Mathematics, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00


Chemistry, 12.12.2020 17:00

English, 12.12.2020 17:00


History, 12.12.2020 17:00




English, 12.12.2020 17:00

English, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00


History, 12.12.2020 17:00


Mathematics, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00

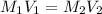 . M stands for molarity of the given substance, and V stands for the volume that the substance occupies.
. M stands for molarity of the given substance, and V stands for the volume that the substance occupies.