Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 23.06.2019 01:10
Volume is a measurement of how fast particles of a substance are moving
Answers: 3

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 1
You know the right answer?
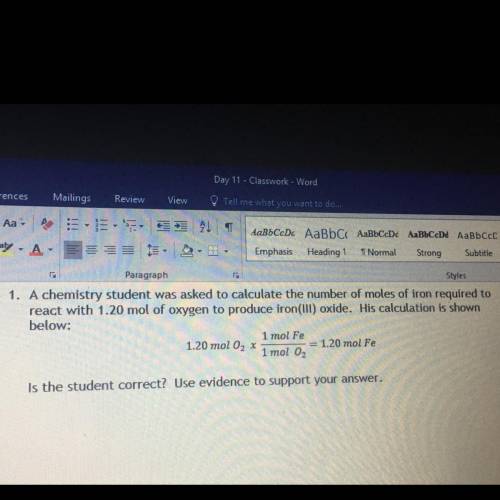
A chemistry student was asked to calculate the number of moles of iron required to react with 1.20 m...
Questions

Mathematics, 23.02.2021 19:30

Mathematics, 23.02.2021 19:30

Computers and Technology, 23.02.2021 19:30

Mathematics, 23.02.2021 19:30






English, 23.02.2021 19:30

Biology, 23.02.2021 19:30

Mathematics, 23.02.2021 19:30



Mathematics, 23.02.2021 19:30

History, 23.02.2021 19:30

Mathematics, 23.02.2021 19:30

Computers and Technology, 23.02.2021 19:30

Mathematics, 23.02.2021 19:30