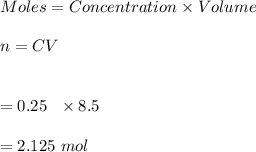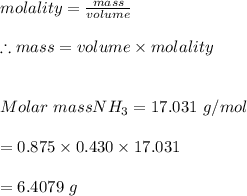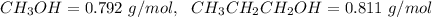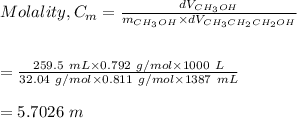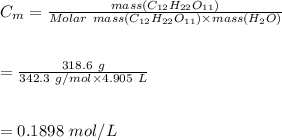Chemistry, 24.04.2020 01:00 robert7248
1. Calculate the number of moles of sulfuric acid that is contained in 250 mL of 8.500 M sulfuric acid solution
2. 7.300 moles of sodium nitrite are needed for a reaction. The solution is 5.450 M. How many mL are needed?
3. What mass (in g) of NH3 must be dissolved in 875 g of methanol to make a 0.430 molal solution?
4. Calculate the molality of a solution that is prepared by mixing 259.5 mL of CH3OH
(d = 0.792 g/mL) and 1387 mL of CH3CH2CH2OH (d = 0.811 g/mL)
5. A solution is prepared by dissolving 318.6 g sucrose (C12H22O11) in 4905 g of water. Determine the molarity of the solution

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
You know the right answer?
1. Calculate the number of moles of sulfuric acid that is contained in 250 mL of 8.500 M sulfuric ac...
Questions





Chemistry, 18.07.2019 23:20



Mathematics, 18.07.2019 23:20


Mathematics, 18.07.2019 23:20

Health, 18.07.2019 23:30

Biology, 18.07.2019 23:30




History, 18.07.2019 23:30


Mathematics, 18.07.2019 23:30


Mathematics, 18.07.2019 23:30