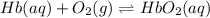Chemistry, 23.04.2020 23:18 lovethenae12
As climbers ascend (go up) a mountain, the amount of oxygen in the atmosphere
decreases. In the body, hemoglobin (Hb) in red blood cells binds with oxygen (02) to
form oxyhemoglobin (HbO2).
This equation can be used to model the equilibrium between hemoglobin , oxygen,
and oxyhemoglobin.
Hb + O2 <--> HbO2
According to Le Chatelier, how would the equilibrium shift as the climbers ascend to
higher altitudes?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3


Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
As climbers ascend (go up) a mountain, the amount of oxygen in the atmosphere
decreases. In the...
decreases. In the...
Questions

History, 26.11.2019 09:31

Mathematics, 26.11.2019 09:31

Mathematics, 26.11.2019 09:31




Mathematics, 26.11.2019 09:31


English, 26.11.2019 09:31


Mathematics, 26.11.2019 09:31





Mathematics, 26.11.2019 09:31


Mathematics, 26.11.2019 09:31


Social Studies, 26.11.2019 09:31