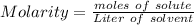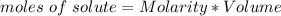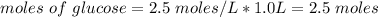Chemistry, 01.01.2020 18:31 vinniemccray70
How many moles of glucose are present in 1.0 liters of a 2.5 m solution of glucose water

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1


Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1
You know the right answer?
How many moles of glucose are present in 1.0 liters of a 2.5 m solution of glucose water...
Questions


Spanish, 24.04.2021 01:00





Mathematics, 24.04.2021 01:00

Mathematics, 24.04.2021 01:00

Biology, 24.04.2021 01:00

Biology, 24.04.2021 01:00




Mathematics, 24.04.2021 01:00

Mathematics, 24.04.2021 01:00




Mathematics, 24.04.2021 01:00