
Chemistry, 23.04.2020 21:31 janighad01
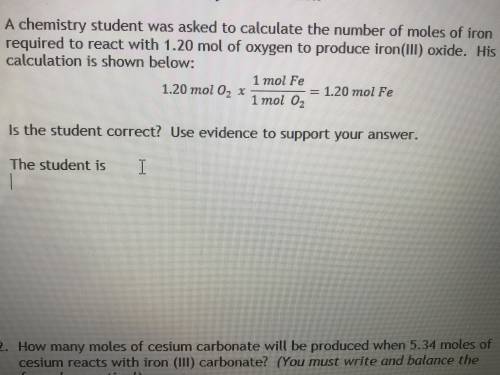
A chemistry student was asked to calculate the number of moles of iron required to react with 1.20 mol of oxygen to produce iron (iii) oxide. his calculation is shown below :
1.20 mol O2 x 1 mol fe / 1 mol O2 = 1.20 mol Fe
Is the student correct? Use evidence to support your answer.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 07:20
Which of the following are acids or bases? 1. sodium hydrogen 2. barium hydroxide solution 3. carbonate solution
Answers: 1
You know the right answer?
A chemistry student was asked to calculate the number of moles of iron required to react with 1.20 m...
Questions

Business, 16.04.2020 13:46



Mathematics, 16.04.2020 13:46


Mathematics, 16.04.2020 13:47

Mathematics, 16.04.2020 13:47


Chemistry, 16.04.2020 13:47

Chemistry, 16.04.2020 13:47


Mathematics, 16.04.2020 13:48



Mathematics, 16.04.2020 13:49



Chemistry, 16.04.2020 13:50

Mathematics, 16.04.2020 13:50

Mathematics, 16.04.2020 14:01



