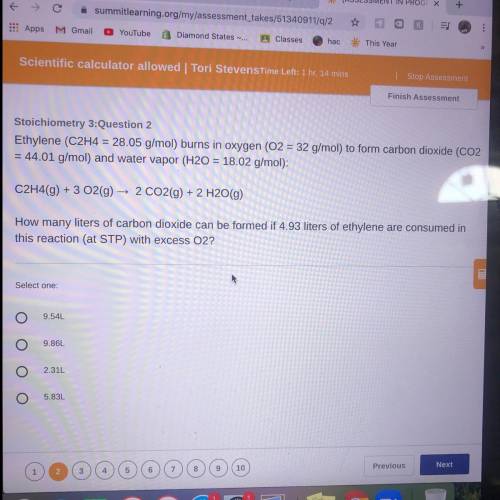
Ethylene (C2H4 = 28.05 g/mol) burns in oxygen (O2 = 32 g/mol) to form carbon dioxide (CO2
= 44...

Chemistry, 23.04.2020 20:23 tchase0616
Ethylene (C2H4 = 28.05 g/mol) burns in oxygen (O2 = 32 g/mol) to form carbon dioxide (CO2
= 44.01 g/mol) and water vapor (H20 = 18.02 g/mol):
C2H4(9) + 3 O2(g) → 2 CO2(g) + 2 H2O(g)
How many liters of carbon dioxide can be formed if 4.93 liters of ethylene are consumed in
this reaction (at STP) with excess O2?
Select one:
9.54L
9.86L
2.31L
5.83L

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 22:00
For a family dinner, jose’s mom baked a loaf of bread. during the meal, his grandmother commented, “this bread is so dense! ” what do you think his grandmother meant by the word dense? you have some ideas regarding density already, but this experiment will enrich your understanding of the relationship between mass, volume and the density of objects. when making measurements in this experiment we will be using si units, the international system of units. the purpose of using si units is to have the ability to communicate and compare data results with other experiments without having to make conversions. measurement symbol si unit length m meter mass kg kilogram volume l liters density kg/l kilograms per liter any calculations that are performed during this experiment will ultimately be reported in scientific notation. recall that scientific notation is extremely important when we have data that is extremely small or large. scientists rely on a standard form of communication to quickly make hypotheses and judgements based on data. what is the density of block a? a0kg/l what is the density of block b? a1kg/l what is the density of block c? a2kg/l what is the density of block d? a3kg/l what is the density of block e? a4kg/l which block is the densest? a5
Answers: 1

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3

Chemistry, 23.06.2019 11:30
The density of e85 fuel is 0.801 g/ml. what is the mass of 1.00 gallon of the fuel? (1 gal. = 3.785 l)
Answers: 3
You know the right answer?
Questions


Mathematics, 15.01.2021 18:50



Mathematics, 15.01.2021 18:50

Mathematics, 15.01.2021 18:50

Social Studies, 15.01.2021 18:50

Business, 15.01.2021 18:50

Mathematics, 15.01.2021 18:50

Social Studies, 15.01.2021 18:50

Mathematics, 15.01.2021 18:50

Mathematics, 15.01.2021 18:50


History, 15.01.2021 18:50


History, 15.01.2021 18:50


Computers and Technology, 15.01.2021 18:50

English, 15.01.2021 18:50

Mathematics, 15.01.2021 18:50



