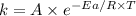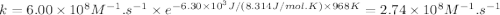Chemistry, 23.04.2020 00:07 oscarsanchez1530
The gas-phase reaction of NO with F2 to form NOF and F has an activation energy of Ea = 6.30 kJ/mol and a frequency factor of A = 6.00×108M−1⋅s−1 . The reaction is believed to be bimolecular:NO(g)+F2(g)→NOF(g)+F(g) What is the rate constant at 695 ∘C ?Express your answer to three significant digits with the appropriate units. For compound units, place a multiplication dot between units (e. g. J⋅mol−1⋅K−1).(I got 2.74*10^8 M-1s-1 but it is wrong. I got a message saying "Enter your answer using dimensions of rate constant units for second-order reaction". Can someone please help me?)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1
You know the right answer?
The gas-phase reaction of NO with F2 to form NOF and F has an activation energy of Ea = 6.30 kJ/mol...
Questions


Computers and Technology, 07.11.2019 09:31

Social Studies, 07.11.2019 09:31

Health, 07.11.2019 09:31

English, 07.11.2019 09:31


History, 07.11.2019 09:31

Mathematics, 07.11.2019 09:31


Mathematics, 07.11.2019 09:31


History, 07.11.2019 09:31

Mathematics, 07.11.2019 09:31




Arts, 07.11.2019 09:31

Mathematics, 07.11.2019 09:31

History, 07.11.2019 09:31

English, 07.11.2019 09:31