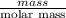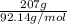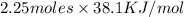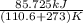Chemistry, 22.04.2020 23:45 phillipselijah2
The heat of vaporization Δ1, of toluene (C6H5CH3) is 38.1 kJ/mol. Calculate the change in entropy AS when 207. g of toluene boils at 1 10.6 °C. Be sure your answer contains a unit symbol. Round your answer to 3 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1


Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1
You know the right answer?
The heat of vaporization Δ1, of toluene (C6H5CH3) is 38.1 kJ/mol. Calculate the change in entropy AS...
Questions

Mathematics, 12.02.2021 23:10


Social Studies, 12.02.2021 23:10

Mathematics, 12.02.2021 23:10

Mathematics, 12.02.2021 23:10


Mathematics, 12.02.2021 23:10



Mathematics, 12.02.2021 23:10




Biology, 12.02.2021 23:10

Mathematics, 12.02.2021 23:10

Mathematics, 12.02.2021 23:10

History, 12.02.2021 23:10



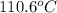 is 223
is 223 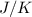 .
.