

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1
You know the right answer?
The hydrolysis of tert-butyl chloride is given in the reaction below: (CH3)3CCl(aq) + H2O(l) → (CH3)...
Questions

Mathematics, 20.01.2021 19:10

Chemistry, 20.01.2021 19:10


Mathematics, 20.01.2021 19:10


English, 20.01.2021 19:10

Business, 20.01.2021 19:10





Mathematics, 20.01.2021 19:10

History, 20.01.2021 19:10

Chemistry, 20.01.2021 19:10

Mathematics, 20.01.2021 19:10


English, 20.01.2021 19:10


Mathematics, 20.01.2021 19:10

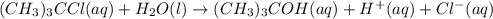
![\text{Rate}=k[(CH_3)_3CCl]^a[H_2O]^b](/tpl/images/0619/8048/0122d.png) ..........(1)
..........(1)

![\text{Rate}=k[(CH_3)_3CCl]](/tpl/images/0619/8048/db534.png) ...........(2)
...........(2)

